In allogeneic hematopoietic stem cell transplantation (HSCT) for high-risk myeloid disorders, the intensity of the conditioning regimen correlates with the risk of relapse, but its benefit may be offset by its risk of toxicity. The addition of increasing doses of TMI to myeloablative conditioning was previously shown to have acceptable toxicity in a phase I trial by our group (PMID 25234438), but the efficacy of this strategy remains uncertain.
From 2017 to 2023, 31 patients with high-risk acute myeloid leukemia (AML, n=20) or myelodysplastic syndrome (MDS, n=5), or chronic myeloid leukemia (CML, n=3) in accelerated phase or blast crisis, were enrolled into a prospective phase II clinical trial of allogeneic HSCT using myeloablative TMI at 9Gy in combination with standard myeloablative fludarabine (160 mg/m2) and targeted IV busulfan (daily exposure of AUC 4800 μM*min over 3 hours) (FluBu4) chemotherapy. TMI was delivered by using volumetric modulated arc therapy (VMAT) at 1.5Gy twice per day for 3 days for a total of 9Gy. The primary objective of the trial was to achieve >50% Disease-Free Survival (DFS) at 1 year.
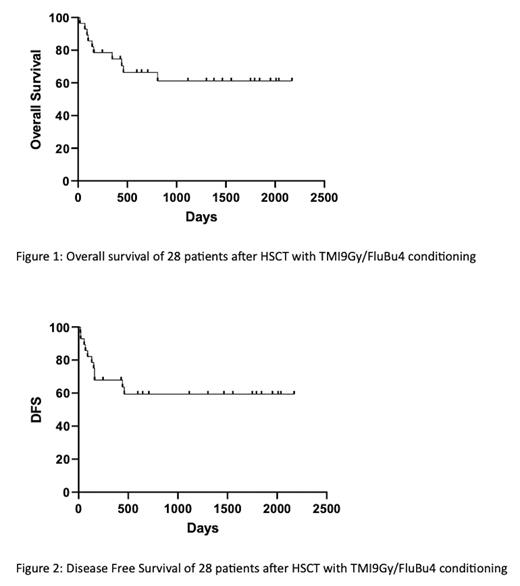
Here, we report the results on 28 patients who received the TMI-based transplant, whereas among the remaining 3, 1 had not started the conditioning yet and 2 became ineligible due to progression of disease and deterioration of performance status, respectively. The study included patients with matched related donors (MRD, n=9) receiving TMI/FluBu4 as a preparative regimen and patients with matched unrelated (MUD, n=13) or 1-antigen mismatched unrelated (MMUD, n=6) donors receiving conditioning with TMI/FluBu4 + rabbit anti-thymocyte globulin (rATG). Graft-versus-host disease (GVHD) prophylaxis was with methotrexate and tacrolimus, and palifermin was used to prevent severe mucositis. Patients' gender ratio was 16 female/12 male; race was Caucasian in 17 patients, African American in 4, Latino in 7; and median age was 53 years (range: 20-68). The median HCT-Comorbidity Index (HCT-CI) was 3 (range: 0-7). All 20 AML patients were at high risk of relapse because of adverse cytogenetic or molecular profile at diagnosis (n=13), or in second complete remission (CR2) (n=2), or with active disease at the time of transplant (n=5). All patients received peripheral blood stem cells (PBSC) (median CD34+ cell dose: 8.5 x 10 6/kg, range: 2.4-19.9) and fully engrafted. The median time to absolute neutrophil count (ANC) and platelet engraftment was 14 (range: day 9-22) and 15 (range: day 9-43) days, respectively. Grade 3-4 extramedullary toxicities were: mucositis in 57% (n=16), nausea/vomiting in 11% (n=3) and diarrhea in 7% (n=2) of the patients. Acute GVHD grade III-IV was seen in 3 patients (10%). Moderate/severe chronic GVHD was observed in 10 patients (36%). With a median follow-up of 1315 days (range: 158-1925 days) for patients alive, the overall 1 year and current overall survival were 73% and 64%, respectively. GVHD-Free Relapse-Free Survival (GRFS) at 1-year was 54%. Of 5 AML patients transplanted with active disease, 3 were alive and in remission at 1 year and at current date, while 2 died of relapsed disease. Of 28 patients in the study, 6 relapsed/progressed (21%) and 5 of them died of the disease (18%); whereas 5 patients (18%) died of transplant-related mortality (TRM) (2 deaths from septic shock, 1 from disseminated toxoplasmosis, 1 from idiopathic pneumonia and 1 from unknown causes). Kaplan Meyer curves for Overall Survival (OS) and DFS are shown in Figure 1 and 2, respectively.
Addition of myeloablative TMI at 9Gy to standard myeloablative targeted FluBu4 was well tolerated and achieved very encouraging results in a group of patients with myeloid malignancies at high risk of relapse (clinicaltrials.gov Identifier: NCT03121014).
Disclosures
Sweiss:Sanofi: Consultancy. Quigley:Recordati Rare Diseases, Inc: Honoraria; Alnylam Pharmaceuticals: Speakers Bureau; Rigel Pharmaceuticals Inc.: Current equity holder in publicly-traded company, Honoraria; Servier Pharmaceuticals: Speakers Bureau; Teva Pharmaceuticals: Research Funding; Mitsubishi: Consultancy; Amgen Pharmaceuticals: Research Funding; Pfizer: Research Funding; AbbVie: Research Funding. Saraf:Agios: Consultancy, Other: Advisory board; Novartis: Consultancy, Other: Advisory board, Research Funding; BEAM Therapeutics: Consultancy, Other: Advisory board; Forma Therapeutics: Consultancy, Other: Advisory board, Research Funding; GBT/Pfizer: Consultancy, Other: Advisory board, Research Funding, Speakers Bureau. Rondelli:Vertex: Other: Steering Committee.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal